- Email us: sales@msbdocs.com
MSB Docs enables an end-to-end smart document solution throughout the GxP spectrum. This, in turn, helps in:
GxP is a general abbreviation for the “good practice” quality guidelines and regulations that were established in the U.S. by the Food and Drug Administration (FDA). These guidelines and regulations ensure that bio/pharmaceutical products under consideration are safe to use and meet their intentional use. Since the quality is not compromised in the industries that directly impact human health, GxP has special guidelines to check whether the products adhere to the quality processes during manufacturing, control, storage, and distribution or not.
In order to ensure that the quality of the goods or services is consistently high, organizations need to focus on the following areas:
Having a way to reconstruct the entire history of a product provides you traceability within the system
The ability to demonstrate the contribution of each person in the product enhances accountability.
Ensuring that there’s a minimum deviation in the promised quality standards maintains integrity.
Keeping the clinical trials and the experimentation carried out in check ensures accuracy.
Conducting routine audits and thorough documentation maintains availability of current data.
Carrying out GxP regulated business processes manually has more chances to deviate organizations from these primary areas. Still, manual processes and paper are widely used in many life science organizations, delaying product launches, and increasing development costs. In other words, leave them behind the competition.

Partner with industry expert that let you carry out document transactions which meets FDA Regulations.
Go Paperless & automate processes to connect the entire agreement process. This increases the process efficiency and productivity, and let you market faster and keep your business moving forward.
Choose a solution that has 21 CFR Part 11 Module in place.

Switch to paperless GxP compliance and realise additional savings, better turnaround time & faster approvals without the “paper”.
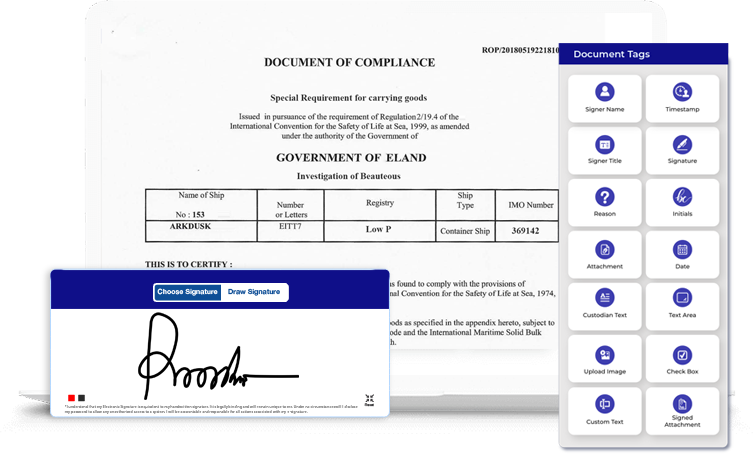
MSB Docs implements a secure and easy to use smart document solution that adheres to GxP regulations. Going paperless is easier than ever with us! Moreover, MSB Docs platform is implemented on compliance with 21 CFR Part 11 requirements on electronic records and signatures. The following industry-designed capabilities that we offer make us stand out from the crowd.
Each MSB Docs account is configured based on the roles, access rights, and permissions within the system to ensure authorized access to the system.
A routing sequence is maintained based on signature level credentialing for operational system checks to enforce permitted sequencing of steps & events.
Time and date stamp along with a defined Audit Trail is made available for each document that is being transacted especially for FDA review and copying.
There are different layers of security and 2-factor authentication for accessing, revising, and changing the data in the document.
A printed name of the signer, date, and time of signing and the meaning (such as review, approval, or responsibility) is associated with each signature.
A custom solution for superfluous collaboration on a document within a team while maintaining regulatory sanctity & the security of the transaction.





MSB Docs lets you carry out the processes while ensuring accuracy, reliability, consistency intended performance, and the ability to discern invalid or altered records.