![]()
- Email us: sales@msbdocs.com
The clinical research stage is resource-intensive, demanding in nature, and possess high risk. So, a fine balance is required for its successful execution. Many pharmaceutical and biotechnology industries are seeking support from CROs (Contract Research Organizations) to fulfill their research-intensive tasks. But unfortunately, this strategy turns out to be more challenging than being an opportunity. Here’s how:
![]()
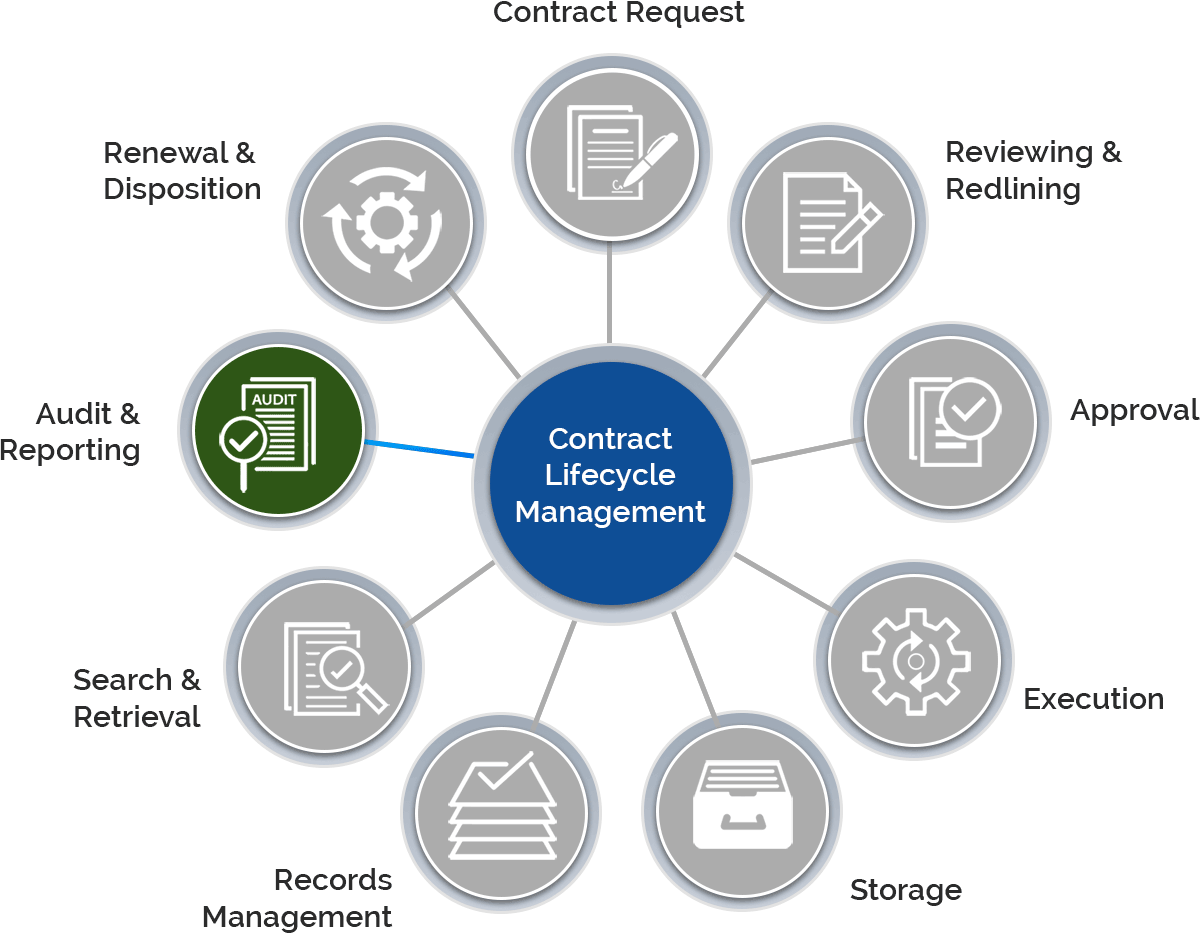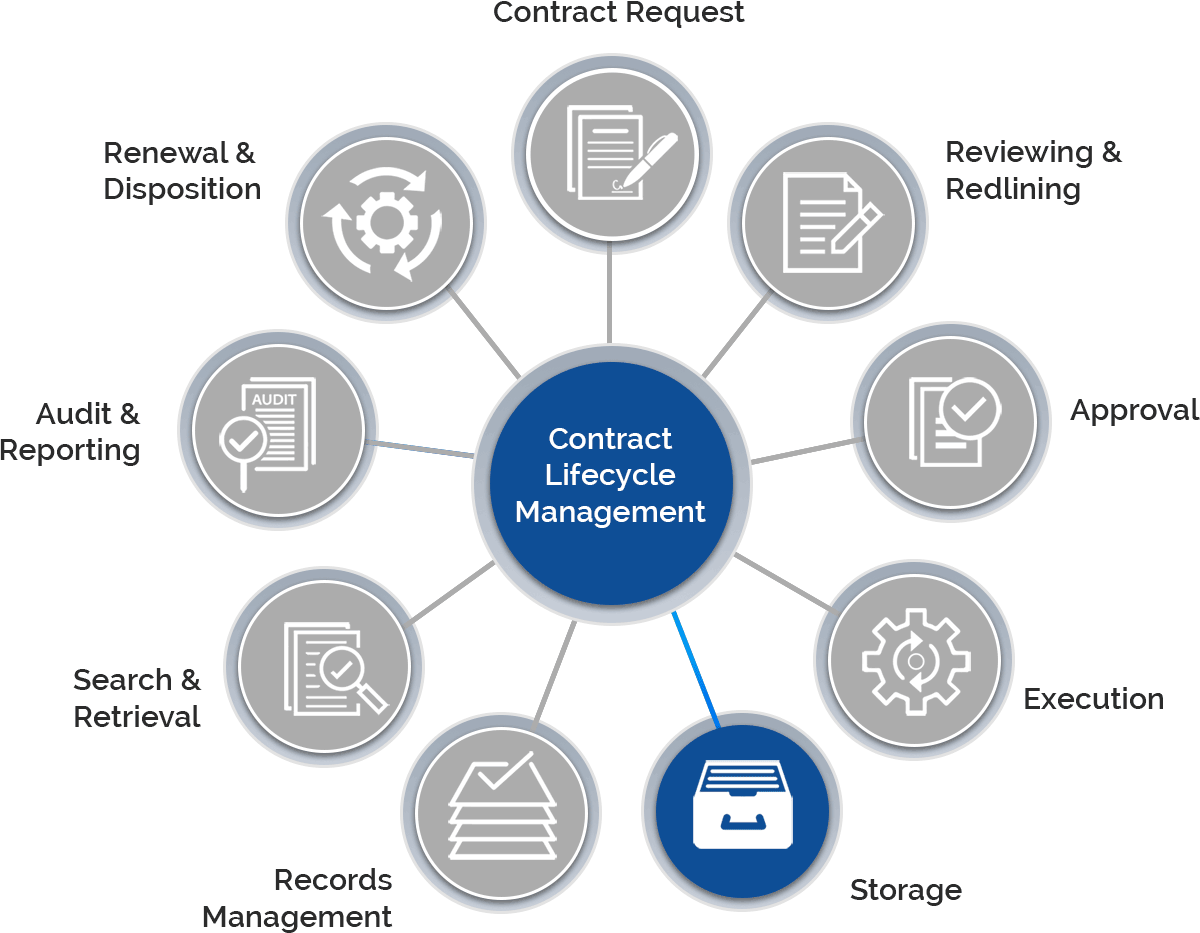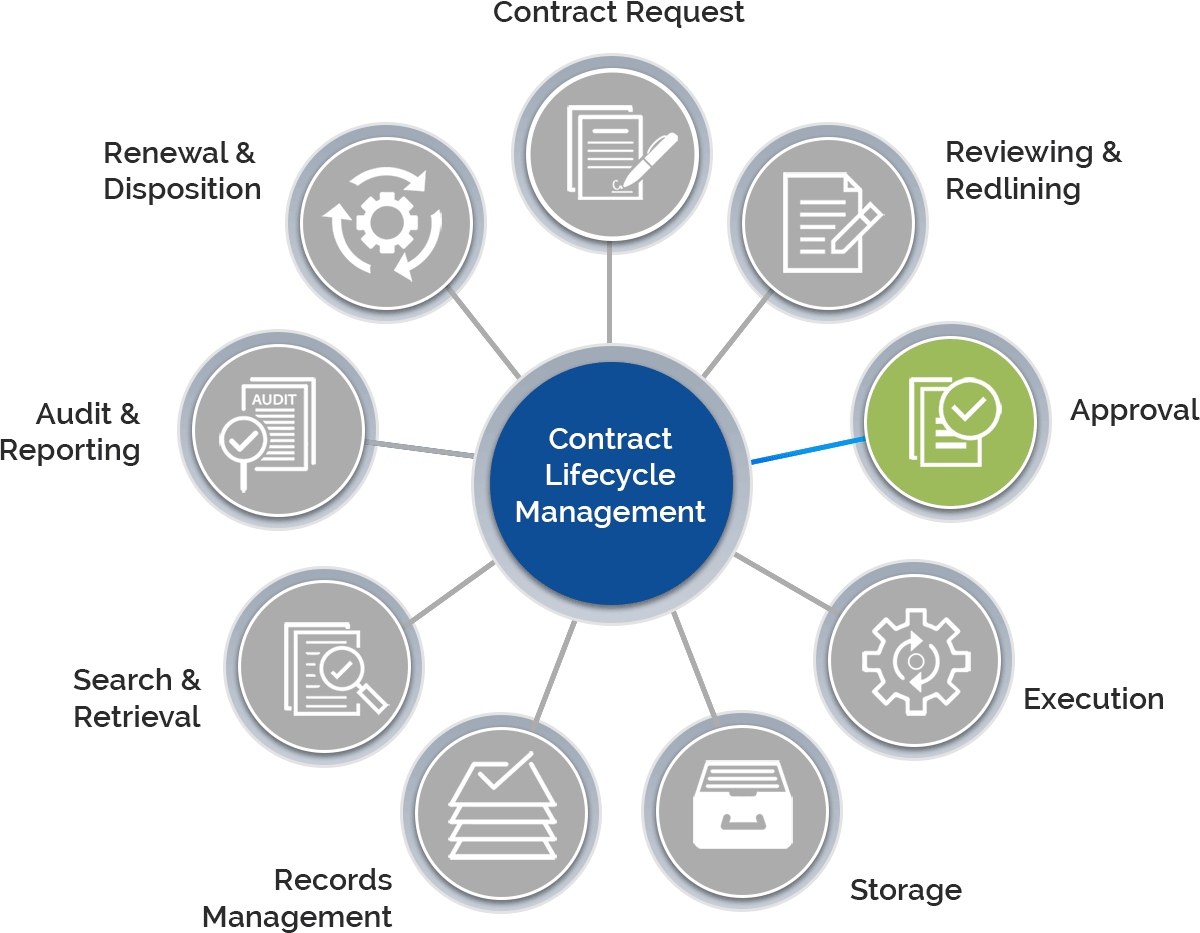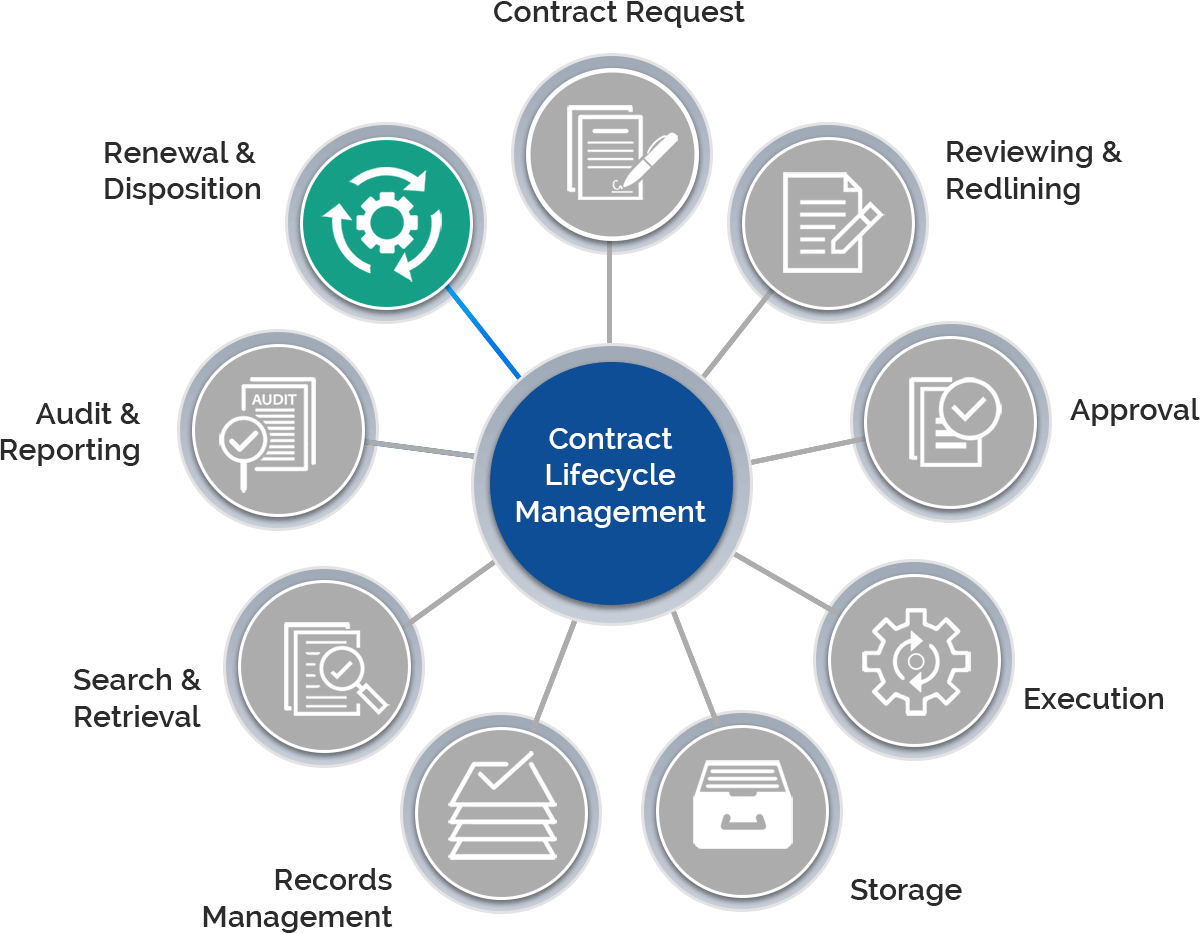
The need to navigate highly regulated promotional programs, meeting ever-increasing compliance, and fulfill the complex supply chains, requires proper document transactions. Depending upon CROs for such contracts that are key to the successful execution of these transactions, it seems difficult with the challenges they hold.

MSB Docs simplifies the processes while keeping up the security and compliance.Here’s how:

Go Paperless

Choose a Digital Solution

Gain Full Control Over Document Transactions









MSB Docs helps in moving a new drug or device from its conception to FDA approval with a lesser need to maintain a staff for these services.
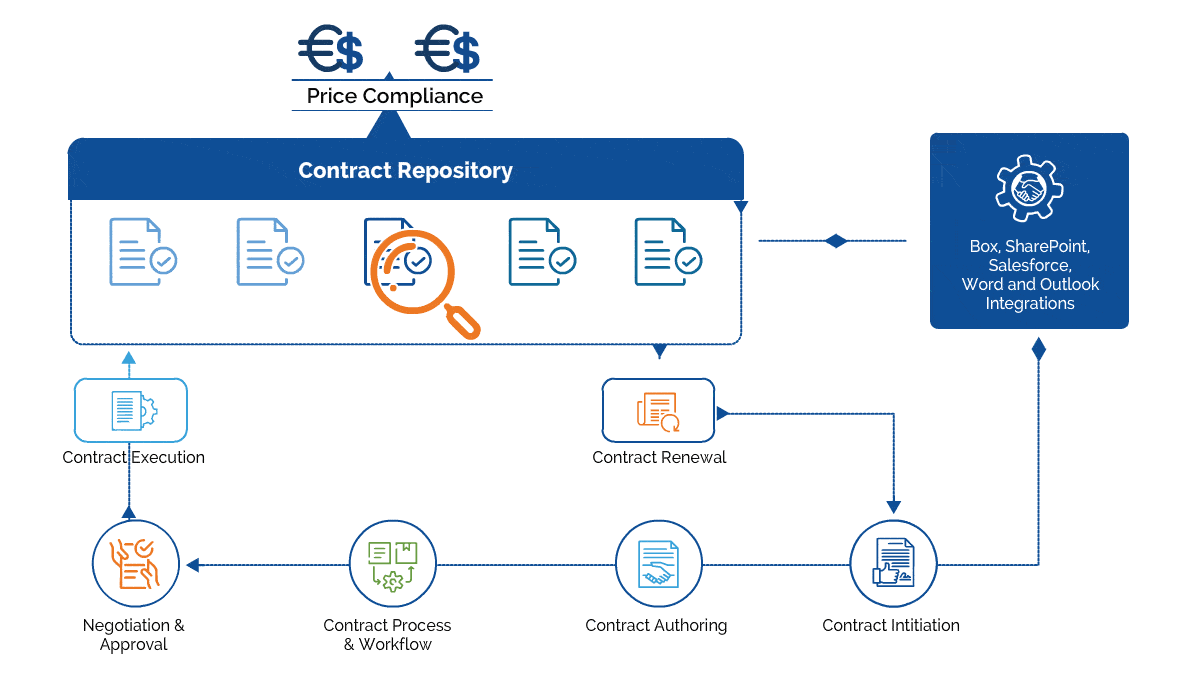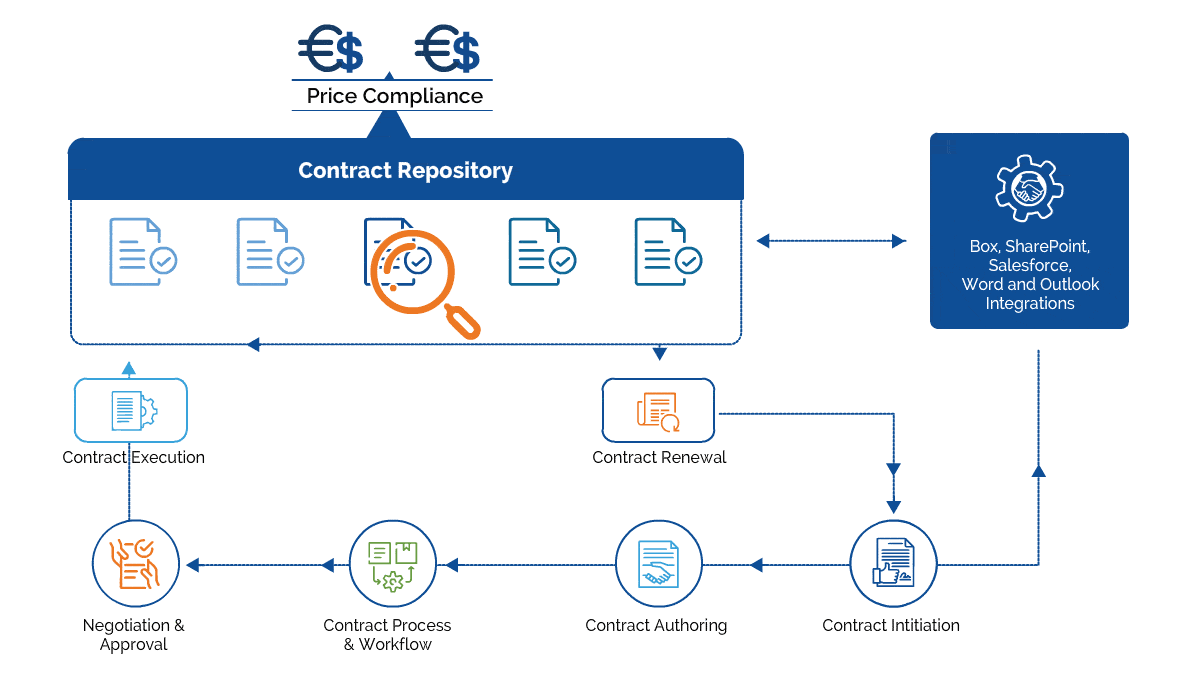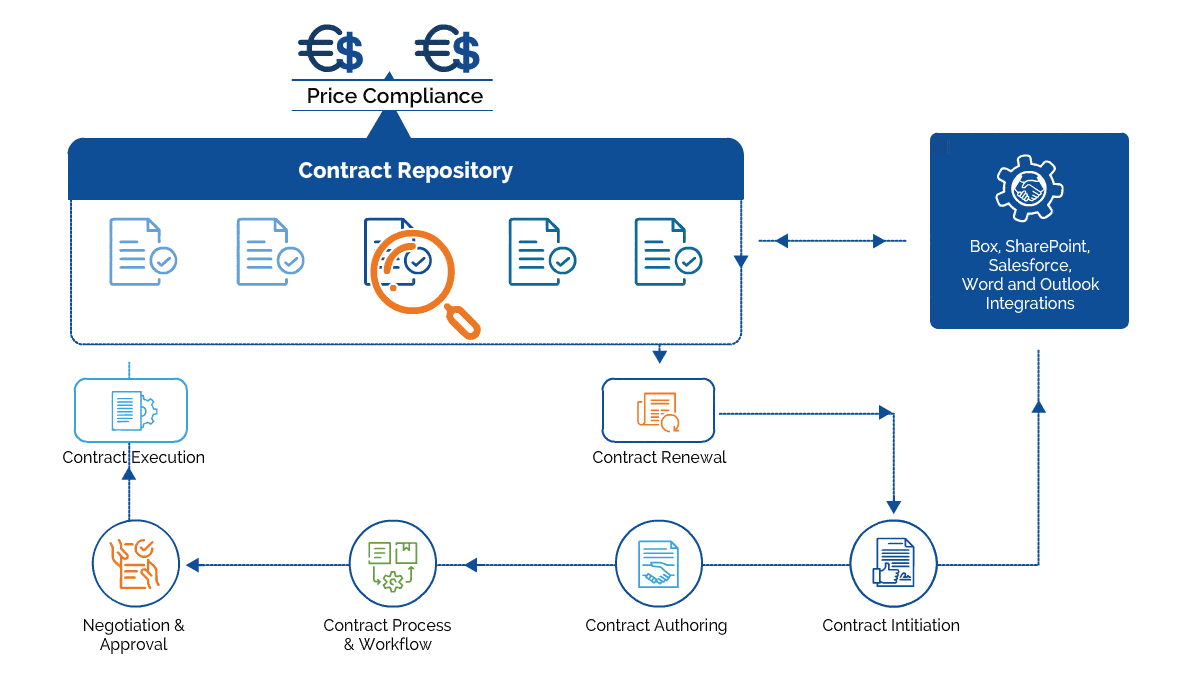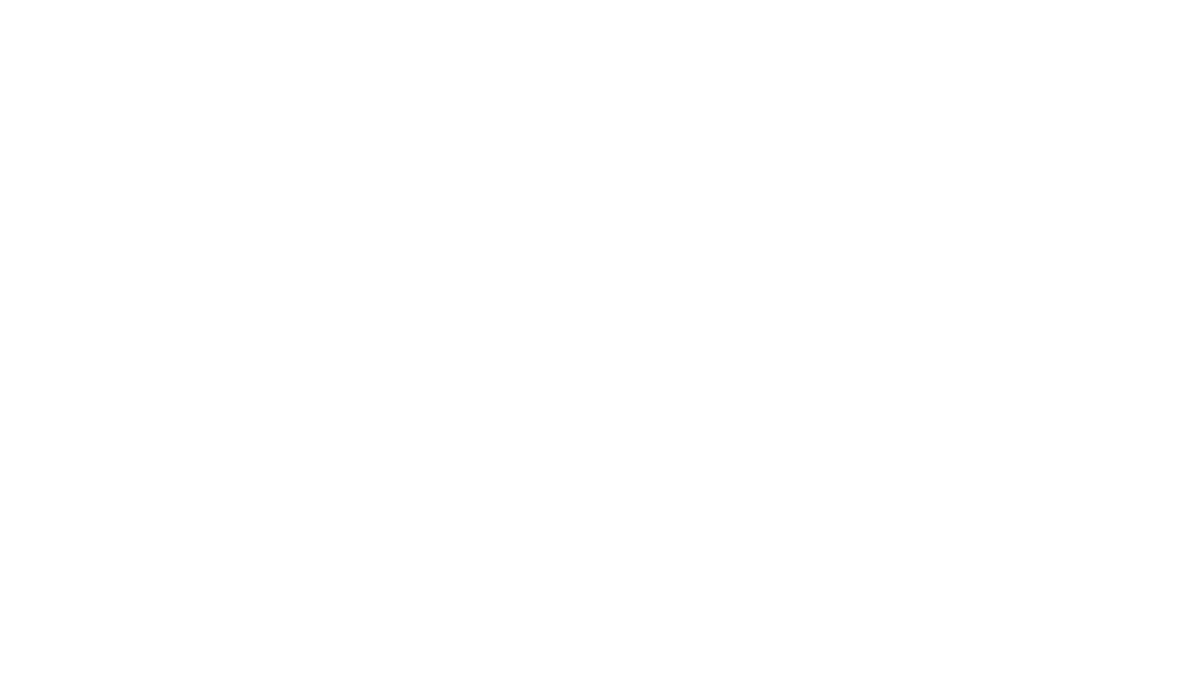
Gain control over the entire contract lifecycle and ensure each process is following the FDA regulations.
Forge collaborative and strategic partnership agreements with CROs
Enforce the use of flattened & fillable forms to meet FDA compliance.
Author all-inclusive Intellectual Property agreements to safeguard patented formulas
Track contractual milestones and identify supplier consolidation opportunities
Create, e-sign, store, and manage all contracts within a centralized repository.
Maintain an audit trail for all the changes made to a contract
Generate reports to increase transparency