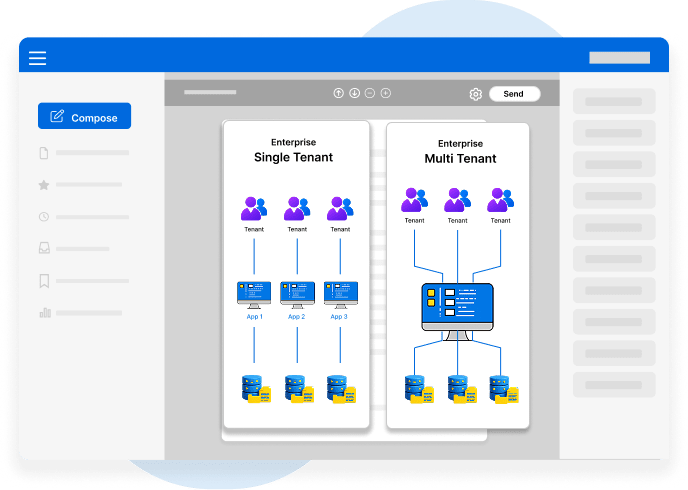
Clinical Trials & Research
Investigator onboarding & credential verification
Digitally sign CVs, GCP training, licenses, and financial disclosures for faster site initiation.
Informed consent forms (ICFs)
Capture remote patient consent on ICFs and HIPAA forms with compliant, timestamped eSignatures.
Patient Consent & Recruitment Documentation
Digitize patient onboarding forms, recruitment logs, and screening checklists with secure,
real-time tracking.
FDA Form 1572 & Clinical Trial Agreements (CTAs)
Digitally sign Form 1572, CTAs, NDAs, and financial agreements with audit-ready compliance.
Clinical trial onboarding documents
Sign IRB approvals, lab certifications, site feasibility, and delegation log digitally.
Protocol Deviation Reporting & Compliance Tracking
eSign deviation reports, CAPAs, and incident logs with traceable, audit-compliant workflows.
Remote monitoring and CRA document uploads
Allow CRAs to upload and sign monitoring documents from remote locations securely.
Mobile data capture for CRAs (Scan & Sign App)
Capture and sign trial data onsite using mobile apps with encrypted sync.
Cross-functional collaboration
Facilitate streamlined workflows between investigators, CRAs, QA teams, and regulatory staff with centralized, compliant documentation accessible in real time.
Regulatory & Compliance
GxP compliance documentation
eSign validation protocols, deviation logs, and change
controls to meet GxP and data integrity standards.
21 CFR Part 11 compliant eSignatures
Apply compliant eSignatures to quality records, SOPs, and electronic batch documentation, with immutable audit trails ensuring traceability and compliance.
Qualified Electronic Signatures (QES)
Enable the highest level of trust and legal enforceability under EU eIDAS, ensuring identity verification and non-repudiation for critical documents.
Computer System Validation (CSV) logs
Digitally sign IQ/OQ/PQ protocols, risk assessments, and system validation records.
FDA Form 483 response documents
eSign CAPA plans, response letters, and supporting documents for post-483 observations.
cGMP inspection reports
Approve and sign cGMP audit findings, internal inspection reports, and follow-up actions.
Regulatory submission forms
Digitally execute forms like IND, NDA, ANDA, and Module 1 submission letters.
Audit trail reports and validation documentation
eSign system audit trails, validation summaries, and deviation tracking logs securely.
SOP acknowledgements and revisions
Collect electronic signatures for SOP approvals, training acknowledgements, and version updates.
Lab & Manufacturing Operations
Lab Accreditation Documentation
Digitally sign audit reports, compliance
certificates, and lab certification documents for regulatory readiness.
Quality control records
eSign test results, analytical data sheets, and COAs to ensure traceable QC documentation.
Batch release forms
Approve batch records, release forms, and QA disposition reports with compliant digital
signatures.
Equipment calibration logs
Sign off calibration certificates, maintenance logs, and verification checklists electronically.
Environmental monitoring checklists
eSign temperature, humidity, and contamination logs to maintain cGMP-compliant facility
conditions.
Production run approvals
Digitally authorize manufacturing start-up checklists, run sheets, and production logs.
In-process inspection sign-offs
Apply eSignatures to IPQA checklists, sampling records, and deviation observations in real time.
Material safety and handling forms
eSign MSDS forms, material transfer records, and hazardous material handling logs securely.
Contracts & Legal
Contract Research Organization (CRO) agreements
eSign CRO contracts, SOWs, and
collaboration terms to accelerate clinical outsourcing workflows.
Vendor and supplier contracts
Digitally execute supply agreements, SLAs, and quality agreements for faster procurement cycles.
Master Service Agreements (MSAs)
Streamline MSAs and addenda with traceable eSignatures and automated approval workflows.
Confidentiality agreements (NDAs)
eSign mutual and one-way NDAs with tamper-proof audit trails and signer authentication.
Licensing & IP agreements
Digitally sign technology transfer, co-development, and patent licensing agreements securely.
Sales & distribution agreements
eSign regional distribution contracts and channel partner agreements with real-time tracking.
Data sharing agreements
Apply eSignatures to inter-institutional data sharing and clinical data access agreements.
Investigator grants & payment contracts
Execute grant agreements, payment terms, and budget approvals for investigator sites digitally.
Training & HR
In-person training records
eSign attendance logs, trainer sign-offs, and training
completion forms for audit readiness.
eLearning sign-offs
Digitally capture signatures on online module completions and assessment acknowledgements.
GMP/GLP compliance certifications
eSign compliance training certificates and competency validation forms for regulatory audits.
Employee onboarding documents
Digitally execute NDAs, offer letters, tax forms, and role acceptance documents.
Continuing education acknowledgements
Capture eSignatures on CE program participation, completion, and recertification logs.
Role-based access documentation
eSign access approvals, system role assignment forms, and permission change logs.
Performance appraisals & HR agreements
Digitally sign appraisal forms, development plans, and workplace policy acknowledgements.
Patient Support & Commercial
Patient assistance program (PAP) forms
eSign PAP applications, eligibility
declarations, and consent forms to expedite access.
Compassionate use approvals
Digitally execute physician requests, approvals, and patient consent for unapproved therapies.
Reimbursement documentation
eSign prior authorizations, payer forms, and benefit verification documents securely.
Enrollment forms for clinical or support programs
Capture eSignatures on patient intake, opt-in, and support program enrollment documents.
Customer service ticket sign-offs
Digitally sign service logs, escalation notes, and issue resolution confirmations.
Field rep daily visit logs
eSign rep visit summaries, HCP interaction records, and sample drop-off forms on mobile.
Call center escalation tracking
Apply eSignatures to escalation approvals, issue reports, and case closure confirmations.